The Philips product defect that has been brought to light earlier this year has been affecting countless patients with sleep apnea & various other lung conditions for years unknowingly. This is a problem that is going to last much longer than the year predicted to replace the 15 Philips CPAP, BIPAP, & home Ventilators. Unfortunately, this is going to cause many with chronic lung illness/issues already to suffer far more than they should.
The 15 listed Philips devices made prior to 04/2021 have been placed on recall due to a Polyester-based polyurethane (PE-PUR) foam, which is used to reduce vibration & sound. It is noted that the PE-PUR foam can degrade into particles small enough to inhale &/or swallow. This foam is also capable of releasing “certain” chemicals which can be inhaled!
According to the FDA the potential risk of exposure to the particulates of the PE-PUR foam “include irritation to the skin, eye, and respiratory tract, inflammatory response, headache, asthma, and toxic or carcinogenic effects to organs, such as kidneys and liver.”
As for the “potential risks of exposure to chemicals released into the device’s air pathway from the PE-PUR foam include headache; dizziness; irritation in the eyes, nose, respiratory tract, and skin; hypersensitivity; nausea/vomiting; and toxic and carcinogenic effects.”
Many of these potential risks were likely put aside as side effects to medications, allergies, worsening of chronic lung illnesses, etc. Not because of lack of insight by medical providers but because the symptom list is not specific, and this was unknowingly happening for years!
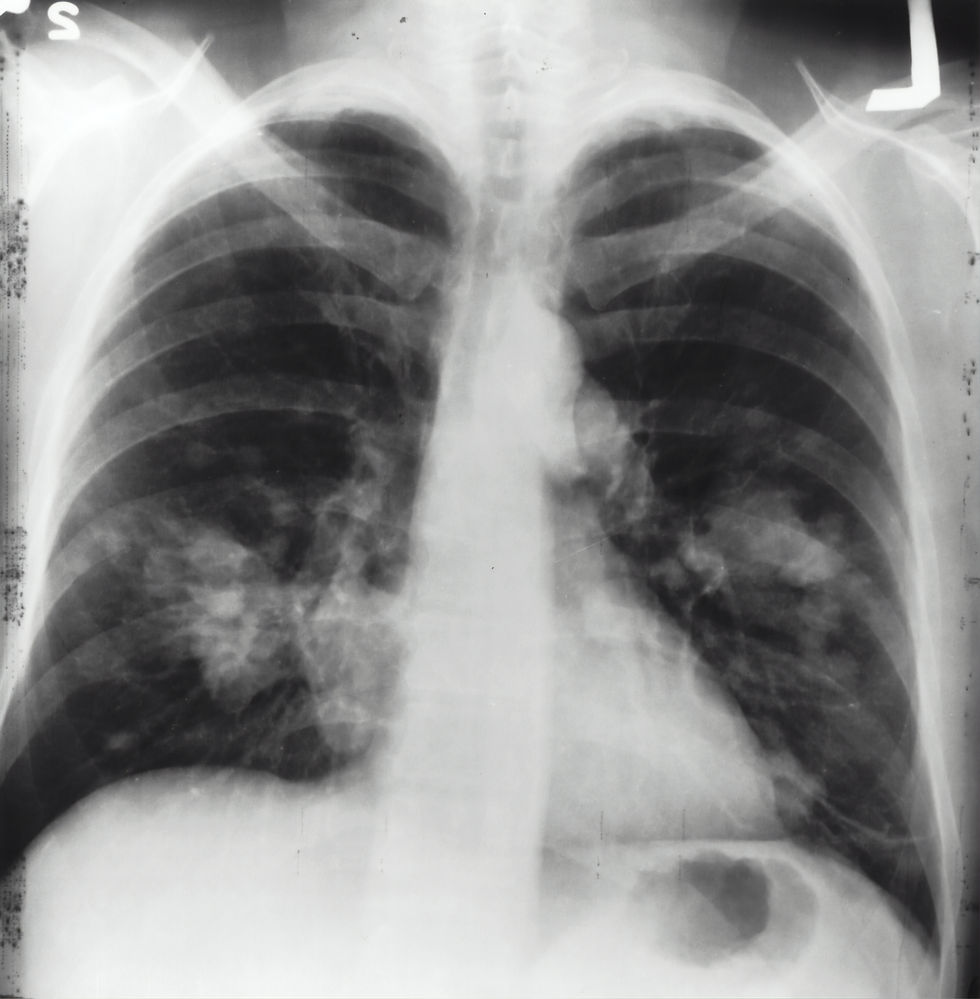
Now that this information is available to the public as well as healthcare professionals it will be much easier to identify the correlation and dig deeper into this being a potential to new (or old) symptoms. Having worked in a procedural unit it is scary/sad how many people come in for a lung biopsy for varying reasons, many with unknown conditions or causes, now I can only wonder… how many of them used one of these devices, how many of them are a victim to this product defect.
What Philips CPAP, BIPAP, Ventilators are affected? How to register a defective device? https://www.usa.philips.com/healthcare/e/sleep/communications/src-update#section_2
FDA Recall Information: https://www.fda.gov/medical-devices/safety-communications/certain-philips-respironics-ventilators-bipap-and-cpap-machines-recalled-due-potential-health-risks
Official Recall Document Philips: https://www.philips.com/c-dam/b2bhc/master/landing-pages/src/update/documents/philips-recall-letter-2021-05-a-2021-06-a.pdf?_ga=2.91682394.1512424789.1631417260-731009723.1631417260&_gl=1*5u0mv*_ga*NzMxMDA5NzIzLjE2MzE0MTcyNjA.*_ga_2NMXNNS6LE*MTYzMTQxNzI1OS4xLjEuMTYzMTQxNzI2OC42MA

Yorumlar